Ceramics are oxides, carbides, nitrides, borides of metal ions. Generally, they are inorganic and non-metallic. Ceramics play an important role in our day-to-day life. Development of ceramics helps to decrease the demand in industries. Compare to other materials ceramics have some unique properties. Ceramics usually withstand high temperature but it has poor mechanical properties.
Here we classify ceramics into five properties:
PHYSICAL PROPERTIES:
Physical properties are identified by its crystal structure and its chemical composition. Generally, Physical properties are identified by simple methods such as odour, colour and physical form of the material (solid, liquid, gas). Other physical properties of ceramics are
- Volume
- Density
- Melting point
- Boiling point
- Heat capacity
- Index of reflection
- Porosity (also we refer pore size)
These are very important parameters for the ceramic material.
The density of ceramics is intermediate between polymers and metals. Crystalline materials have high density than non-crystalline materials. Generally, ceramic particles are fine and coarse. We determine the above all properties with the particle sizes of the material. Fine particles are there in a given ceramics then the volume increases because of less void space hence, density will be high, then it can withstand the heat capacity too. Likewise, reflection is also better in fine particles. The coarse particles are vice versa.., they have more void spaces and less volume hence the density will be less. Coarse particles conduct heat easily because coarse particles have fewer grain boundaries compared to fine particles. Similarly, porosity is less in fine particles compared to coarse particles.
MECHANICAL PROPERTIES:
Mechanical properties generally describe the strength of the material. It is important in structural and building materials. Particle size determines the physical properties. Likewise here stress and strain determine the strength of the material. Ceramics are generally brittle or ductile in nature. If we apply the load, the material will deform up to the material can withstand the load. Above that the material breaks without neck formation this is elastic deformation. This is the brittle type. But in ductile material, when we apply to load the neck forms before failure. This is both elastic and plastic deformation.
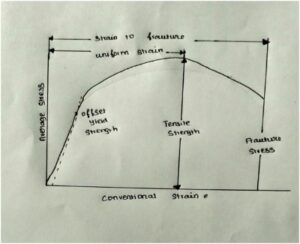
Some of the mechanical properties are:
- compressive strength
- shear strength
- fracture toughness
- elasticity or plasticity
- ductility
- hardness
when we apply stress, strain develops. Compressive strength, tensile strength and toughness also determined by stress and strain.
CHEMICAL PROPERTIES:
Ceramics are bonded together by an ionic or covalent bond. Basically, these bonds result in good chemical resistance but have the low thermal expansion, high melting point and hardness. Ceramics are generally
- alkali attack resistance
- acid attack resistance
- corrosion resistances
Advanced ceramics ( fine ceramics) possess good chemical stability. If the material is subjected to chemically soluble then it is used in a limited range of applications. Chemicals can corrode the materials, even water is corrosive to many metals. In order to check whether the material is chemical resistant or not, the material should be dipped in chemicals ( hydrochloric acid, hydrofluoric acid, sulphuric acid, nitric acid ). If it is chemical resistant the material should not corrode or soluble in chemicals. In ceramics, alumina and silicon carbide posses high chemical resistant material, which means they are low chemical solubility. Though ceramics are resistant to corrosion, it is used for many applications in industries. To increase chemical resistant in ceramics silica should be added.
THERMAL PROPERTIES:
On heating, ceramics expand this is generally known as thermal expansion. Thermal expansion is due to conduction of heat. From this, we know that ceramics conduct heat but withstand high temperature. Major thermal properties of ceramics are
- insulating properties
- semi-conducting properties
- thermal conductivity
- thermal expansion
- thermal shock resistance
Thermal conduction is varied due to the certain factors. These factors are internal porosity, grain boundaries and impurities. By controlling these factors we can increase or decrease the thermal conductivity. Conduction is due to the movement of electrons. If the grain boundaries and porosity is less then the conduction will be high. Aluminium nitride and silicon carbide have a high level of thermal conductivity and metals to have high thermal conductivity. Zircon blocks have low thermal conductivity hence it is used in kiln walls. Metals conduct.
ELECTRICAL PROPERTIES:
Ceramics are generally bad conductors. But fine ceramics and porcelain are insulators that do not conduct electricity. Semiconductor ceramics can also be developed to conduct electricity depending upon the voltage applied and their temperature. Some ceramics (cubic boron nitride) are good conductors of electricity. In ceramics, the ionic bonds tightly bond the molecules hence it does not allow the electrons to flow. If there is a flow of free electrons then the material can conduct electricity.
Some of the electrical properties of ceramics materials are
- dielectric strength
- electrical conductivity
- insulating properties
- semiconducting properties
- superconducting properties
- dielectric constant
OPTICAL PROPERTIES:
Advanced ceramics are fine Particles in nature. They possess microscopic grain boundaries and microstructure pores. This diffuses the light and it is difficult to transmit the light. Single crystals have no grain boundaries or pores hence light can easily pass through. Light can also pass through in fine ceramics if we minimizing pores and grain boundary (increase the size of pores and grain boundaries). In addition, fine ceramics have ferromagnetic and semiconductive properties. Because of these properties, there is a change in fluorescence, phosphorescence due to its interactions with light and electric field.
Translucent is the major optical property in ceramics. Light has electromagnetic waves, infrared radiation etc..,. The material with good optical property has an increasing need in the military field as they have high strength and have the capacity to transmit light. These materials are used in the field of transparent armour, high-speed missiles.
In ceramics, Alumina is the good translucent material. Alumina is a transparent enough material and it is used in high-pressure sodium street lamps. For the past twenty years, various types of transparent ceramics have been developed for many applications.
These are all the basic properties of ceramic material. Now we are going to see about the properties of clay.
Clays are generally termed as hydro aluminosilicate. It combines with one or more minerals with the traces of silica, organic impurities and metal oxides.
CLAY COMPOSITION:
Clay has three various compositions.
- Mineralogical composition
- Physical composition
- Chemical composition
Particle size distribution and shapes of minerals are the physical composition. Mineralogical composition determines the amount and type of minerals present.
PROPERTIES OF CLAY:
The properties of clays are
- Plasticity
- Dry strength
- Shrinkage
- Permeability
- Moisture content
- Flow characteristics
- Stability
Clays are classified as primary and secondary clays. Primary clays remain where the formation takes place and the secondary clays transported from where it forms to some other places. Due to transportation, attrition takes place and the size of the particles is reduced. Also, during transportation, water and organic impurities present in the clay increases. Thus, the secondary clay has more plasticity than primary clays. Depends upon the impurities present and water present plasticity determines.
Clays are extracted as lumps from the rocks then it is subjected to weathering. When the lumps are subjected to summering and wintering, cracks forms and then the size reduced. This type of crushed particles is very smooth hence plasticity is high. If plasticity of the material is high then the strength will also be high. Because fine particles have more strength than the coarser particles. Strength also depends upon the density of the clay.
Shrinkage is of two categories: linear shrinkage and volume shrinkage. If we take clay as powders, on heating shrinkage occurs this is meant by volume shrinkage. If we make the clay into pellets, then the water present in it is evaporated during heating. In this, we measure both volume shrinkage and linear shrinkage. During heating, the clay shrinks and gives more strength compared to the green strength.
If the moisture content is more in fine particles, water is very difficult to evaporate during heating. Hence it gives more force to remove the water content. Thus, the strength will be high and the shrinkage is more in fine particles. Moisture content determines the strength, shrinkage and plasticity.
Flow properties of clay:
Water mixed with clay gives a number of types of flow, depending on the nature of clay and also the additives add to the water.
To study the flow of liquids we plot a graph between the shearing stress and rate of shear. Some clay suspension can flow like a liquid. They behave rarely as a homogeneous liquid, and this is called as a Newtonian viscous flow. The Newtonian viscous flow gives a straight line in the graph. This shows that the viscosity or fluidity is independent of the rate of shear. Most of the clays, when mixed with water, has high viscosity and decreases the rate of shear. It should be a curve or a line which does not pass through the origin. This is known as plastic flow. When deflocculates are added to the mixture the dilatant curve is obtained, which occurs near the origin.

properties of ceramics
When we kept the mixture for sometimes and not disturbing it, it stiffens and becomes the gel. On stirring it to retain to its original state. This is known as thixotropy flow. Here the curve obtained is that increasing and decreasing rate of shear. One more curve negative thixotropy also obtained near the thixotropy.
Here we discussed the properties of clays. The property should be the important parameter for any applications and for further use. Depends upon the property, the products will differ.


